what happens to bonds between molecules when a substance melts
Intermolecular Forces |
|---|
Intermolecular Forces
The molecule is the smallest appreciable grouping of uniquely bonded atoms that stand for the composition, configuration and characteristics of a pure chemical compound. Our main focus upwardly to this indicate has been to discover and describe the ways in which atoms bond together to form molecules. Since all appreciable samples of compounds and mixtures contain a very large number of molecules (ca.!020), we must as well concern ourselves with interactions between molecules, as well every bit with their individual structures. Indeed, many of the physical characteristics of compounds that are used to place them (eastward.g. boiling points, melting points and solubilities) are due to intermolecular interactions.
All atoms and molecules accept a weak attraction for i another, known equally van der Waals allure. This bonny force has its origin in the electrostatic attraction of the electrons of ane molecule or atom for the nuclei of another. If there were no van der Waals forces, all matter would exist in a gaseous country, and life as we know it would not be possible. Information technology should be noted that there are also smaller repulsive forces between molecules that increase rapidly at very small-scale intermolecular distances.
Boiling & Melting Points |
|---|
Boiling Points
For general purposes information technology is useful to consider temperature to be a measure out of the kinetic energy of all the atoms and molecules in a given organization. As temperature is increased, there is a respective increase in the vigor of translational and rotation motions of all molecules, as well equally the vibrations of atoms and groups of atoms within molecules. Experience shows that many compounds exist normally as liquids and solids; and that fifty-fifty low-density gases, such as hydrogen and helium, can exist liquified at sufficiently low temperature and high pressure level. A clear conclusion to be drawn from this fact is that intermolecular attractive forces vary considerably, and that the boiling signal of a compound is a measure of the strength of these forces. Thus, in guild to intermission the intermolecular attractions that concord the molecules of a compound in the condensed liquid state, it is necessary to increment their kinetic energy past raising the sample temperature to the feature boiling point of the chemical compound.
The post-obit table illustrates some of the factors that influence the strength of intermolecular attractions. The formula of each entry is followed past its formula weight in parentheses and the boiling betoken in degrees Celsius. First in that location is molecular size. Big molecules have more electrons and nuclei that create van der Waals attractive forces, so their compounds usually have higher boiling points than similar compounds made up of smaller molecules. It is very of import to apply this rule only to like compounds. The examples given in the offset two rows are similar in that the molecules or atoms are spherical in shape and exercise non have permanent dipoles. Molecular shape is also important, as the 2d group of compounds illustrate. The upper row consists of roughly spherical molecules, whereas the isomers in the lower row have cylindrical or linear shaped molecules. The attractive forces between the latter group are by and large greater. Finally, permanent molecular dipoles generated by polar covalent bonds result in even greater attractive forces betwixt molecules, provided they accept the mobility to line up in appropriate orientations. The last entries in the table compare non-polar hydrocarbons with equal-sized compounds having polar bonds to oxygen and nitrogen. Halogens also form polar bonds to carbon, but they too increase the molecular mass, making it difficult to distinguish among these factors.
Boiling Points (ºC) of Selected Elements and Compounds | ||||
|---|---|---|---|---|
| Increasing Size | ||||
| Diminutive | Ar (40) -186 | Kr (83) -153 | Xe (131) -109 | |
| Molecular | CH4 (sixteen) -161 | (CH3)4C (72) 9.5 | (CHthree)fourSi (88) 27 | CCliv (154) 77 |
| Molecular Shape | ||||
| Spherical: | (CHthree)fourC (72) 9.5 | (CH3)2CCl2 (113) 69 | (CH3)3CC(CH3)3 (114) 106 | |
| Linear: | CHthree(CH2)3CH3 (72) 36 | Cl(CH2)3Cl (113) 121 | CHthree(CH2)6CH3 (114) 126 | |
| Molecular Polarity | ||||
| Not-polar: | HtwoC=CH2 (28) -104 | F2 (38) -188 | CH3C≡CCH3 (54) -32 | CF4 (88) -130 |
| Polar: | HtwoC=O (30) -21 | CHthreeCH=O (44) 20 | (CH3)iiiN (59) 3.5 | (CH3)2C=O (58) 56 |
| HC≡N (27) 26 | CHthreeC≡Northward (41) 82 | (CH2)3O (58) fifty | CHiiiNO2 (61) 101 | |
The melting points of crystalline solids cannot be categorized in equally simple a fashion as boiling points. The altitude between molecules in a crystal lattice is small and regular, with intermolecular forces serving to constrain the move of the molecules more severely than in the liquid land. Molecular size is important, but shape is also disquisitional, since individual molecules need to fit together cooperatively for the attractive lattice forces to be large. Spherically shaped molecules more often than not have relatively high melting points, which in some cases approach the boiling point. This reflects the fact that spheres tin can pack together more closely than other shapes. This structure or shape sensitivity is one of the reasons that melting points are widely used to identify specific compounds. The data in the following tabular array serves to illustrate these points.
| Compound | Formula | Boiling Bespeak | Melting Point |
|---|---|---|---|
| pentane | CHthree(CHtwo)3CH3 | 36ºC | –130ºC |
| hexane | CH3(CH2)fourCH3 | 69ºC | –95ºC |
| heptane | CH3(CHii)5CHthree | 98ºC | –91ºC |
| octane | CHthree(CH2)6CH3 | 126ºC | –57ºC |
| nonane | CHiii(CHii)viiCH3 | 151ºC | –54ºC |
| decane | CHiii(CH2)8CH3 | 174ºC | –30ºC |
| tetramethylbutane | (CHiii)threeC-C(CHiii)3 | 106ºC | +100ºC |
Observe that the humid points of the unbranched alkanes (pentane through decane) increase rather smoothly with molecular weight, but the melting points of the fifty-fifty-carbon bondage increase more than than those of the odd-carbon chains. Even-membered chains pack together in a uniform mode more than compactly than do odd-membered chains. The last compound, an isomer of octane, is nearly spherical and has an uncommonly high melting indicate (just 6º below the boiling point).
Hydrogen Bonding |
|---|
Hydrogen Bonding
The virtually powerful intermolecular force influencing neutral (uncharged) molecules is the hydrogen bond. If we compare the humid points of methyl hydride (CH4) -161ºC, ammonia (NH3) -33ºC, water (H2O) 100ºC and hydrogen fluoride (HF) 19ºC, we encounter a greater variation for these similar sized molecules than expected from the data presented above for polar compounds. This is shown graphically in the following chart. Well-nigh of the simple hydrides of group Iv, V, VI & 7 elements display the expected rise in boiling betoken with molecular mass, but the hydrides of the most electronegative elements (nitrogen, oxygen and fluorine) accept abnormally high boiling points for their mass.
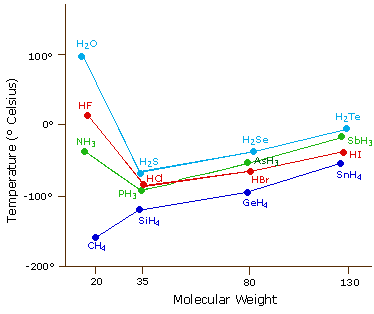
The exceptionally strong dipole-dipole attractions that crusade this behavior are chosen the hydrogen bond. Hydrogen forms polar covalent bonds to more electronegative atoms such as oxygen, and because a hydrogen atom is quite modest, the positive end of the bond dipole (the hydrogen) tin can approach neighboring nucleophilic or bones sites more closely than can other polar bonds. Coulombic forces are inversely proportional to the 6th power of the distance between dipoles, making these interactions relatively stiff, although they are notwithstanding weak (ca. 4 to five kcal per mole) compared with most covalent bonds. The unique backdrop of water are largely due to the stiff hydrogen bonding that occurs betwixt its molecules. In the following diagram the hydrogen bonds are depicted as magenta dashed lines.
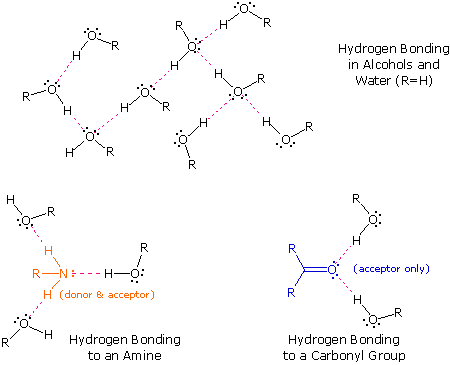
The molecule providing a polar hydrogen for a hydrogen bond is chosen a donor. The molecule that provides the electron rich site to which the hydrogen is attracted is chosen an acceptor. Water and alcohols may serve as both donors and acceptors, whereas ethers, aldehydes, ketones and esters can office just as acceptors. Similarly, primary and secondary amines are both donors and acceptors, but tertiary amines function only equally acceptors. Once y'all are able to recognize compounds that can exhibit intermolecular hydrogen bonding, the relatively high boiling points they exhibit get understandable. The data in the following table serve to illustrate this point.
| Compound | Formula | Mol. Wt. | Boiling Betoken | Melting Point |
|---|---|---|---|---|
| dimethyl ether | CHiiiOCH3 | 46 | –24ºC | –138ºC |
| ethanol | CHiiiCH2OH | 46 | 78ºC | –130ºC |
| propanol | CHiii(CH2)2OH | 60 | 98ºC | –127ºC |
| diethyl ether | (CHthreeCHii)2O | 74 | 34ºC | –116ºC |
| propyl amine | CHiii(CH2)2NH2 | 59 | 48ºC | –83ºC |
| methylaminoethane | CH3CH2NHCHthree | 59 | 37ºC | |
| trimethylamine | (CH3)3N | 59 | 3ºC | –117ºC |
| ethylene glycol | HOCH2CHiiOH | 62 | 197ºC | –13ºC |
| acetic acid | CH3CO2H | 60 | 118ºC | 17ºC |
| ethylene diamine | H2NCH2CH2NH2 | 60 | 118ºC | 8.5ºC |
Alcohols boil considerably higher than comparably sized ethers (first two entries), and isomeric 1º, 2º & 3º-amines, respectively, show decreasing boiling points, with the ii hydrogen bonding isomers being substantially higher boiling than the 3º-amine (entries five to seven). Too, O–H---O hydrogen bonds are clearly stronger than Northward–H---Northward hydrogen bonds, equally we see by comparing propanol with the amines. 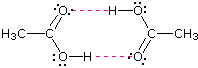 As expected, the presence of ii hydrogen bonding functions in a chemical compound raises the boiling point even further. Acetic acid (the 9th entry) is an interesting example. A dimeric species, shown on the right, held together by ii hydrogen bonds is a major component of the liquid state. If this is an accurate representation of the composition of this compound then we would expect its boiling point to exist equivalent to that of a C4H8O4 compound (formula weight = 120). A suitable approximation of such a compound is found in tetramethoxymethane, (CHthreeO)4C, which is actually a bit larger (formula weight = 136) and has a boiling point of 114ºC. Thus, the dimeric hydrogen bonded structure appears to be a proficient representation of acetic acrid in the condensed country.
As expected, the presence of ii hydrogen bonding functions in a chemical compound raises the boiling point even further. Acetic acid (the 9th entry) is an interesting example. A dimeric species, shown on the right, held together by ii hydrogen bonds is a major component of the liquid state. If this is an accurate representation of the composition of this compound then we would expect its boiling point to exist equivalent to that of a C4H8O4 compound (formula weight = 120). A suitable approximation of such a compound is found in tetramethoxymethane, (CHthreeO)4C, which is actually a bit larger (formula weight = 136) and has a boiling point of 114ºC. Thus, the dimeric hydrogen bonded structure appears to be a proficient representation of acetic acrid in the condensed country.
A related principle is worth noting at this betoken. Although the hydrogen bond is relatively weak (ca. four to 5 kcal per mole), when several such bonds exist the resulting structure can be quite robust. The hydrogen bonds between cellulose fibers confer swell forcefulness to wood and related materials.
| |
|---|
Backdrop of Crystalline Solids |
|---|
Melting Points
| Most organic compounds have melting points below 200 ºC. Some decompose before melting, a few sublime, merely a bulk undergo repeated melting and crystallization without any change in molecular structure. When a pure crystalline compound is heated, or a liquid cooled, the change in sample temperature with time is roughly compatible. However, if the solid melts, or the liquid freezes, a discontinuity occurs and the temperature of the sample remains abiding until the phase change is complete. This beliefs is shown in the diagram on the right, with the green segment representing the solid phase, light blue the liquid, and reddish the temperature invariant liquid/solid equilibrium. For a given chemical compound, this temperature represents its melting bespeak (or freezing point), and is a reproducible constant as long equally the external pressure does not change. The length of the horizontal portion depends on the size of the sample, since a quantity of oestrus proportional to the heat of fusion must be added (or removed) before the phase change is complete. Now information technology is well known that the freezing point of a solvent is lowered past a dissolved solute, due east.g. brine compared with water. If ii crystalline compounds (A & B) are thoroughly mixed, the melting betoken of that mixture is commonly depressed and broadened, relative to the feature sharp melting point of each pure component. This provides a useful means for establishing the identity or non-identity of two or more compounds, since the melting points of numerous solid organic compounds are documented and commonly used as a test of purity. | 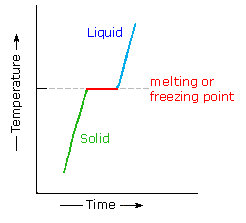 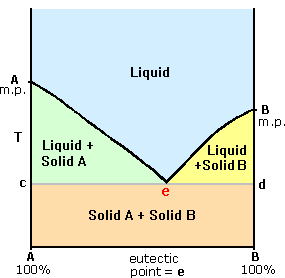 |
| An interesting merely less common mixed system involves molecular components that form a tight complex or molecular compound, capable of existing as a discrete species in equilibrium with a liquid of the same composition. Such a species ordinarily has a sharp congruent melting point and produces a phase diagram having the appearance of 2 side by side eutectic diagrams. An instance of such a system is shown on the right, the molecular chemical compound being represented as A:B or C. I such mixture consists of α-naphthol, 1000.p. 94 ºC, and p-toluidine, k.p. 43 ºC. The A:B complex has a melting point of 54 ºC, and the phase diagram displays two eutectic points, the kickoff at l ºC, the second at thirty ºC. Molecular complexes of this kind ordinarily have a 50:50 stoichiometry, every bit shown, just other integral ratios are known. |  |
Polymorphism
Polymorphs of a compound are different crystal forms in which the lattice organization of molecules are dissimilar. These distinct solids normally have unlike melting points, solubilities, densities and optical backdrop. Many polymorphic compounds have flexible molecules that may assume different conformations, and 10-ray exam of these solids shows that their crystal lattices impose certain conformational constraints. When melted or in solution, different polymorphic crystals of this kind produce the same apace equilibrating mixture of molecular species. Polymorphism is like to, merely distinct from, hydrated or solvated crystalline forms. Information technology has been estimated that over 50% of known organic compounds may be capable of polymorphism.
The ribofuranose tetraacetate, shown at the upper left beneath, was the source of an early puzzle involving polymorphism. The compound was get-go prepared in England in 1946, and had a melting point of 58 ºC. Several years later the same material, having the aforementioned melting point, was prepared independently in Germany and the United States. The American chemists then constitute that the melting points of their early preparations had risen to 85 ºC. Eventually, information technology became apparent that whatsoever laboratory into which the higher melting form had been introduced was no longer able to make the lower melting form. Microscopic seeds of the stable polymorph in the environs inevitably directed crystallization to that stop. X-ray diffraction information showed the lower melting polymorph to be monoclinic, space group P2. The higher melting form was orthorhombic, infinite group P2iii121.
Polymorphism has proven to be a critical factor in pharmaceuticals, solid country pigments and polymer manufacture. Some examples are described beneath.
 |  | Acetaminophen is a mutual analgesic (e.g. Tylenol). It is ordinarily obtained every bit monoclinic prisms (upper picture) on crystallization from water. A less stable orthorhombic polymorph, having better concrete properties for pressing into tablets, is shown beneath the commencement. Quinacridone is an of import pigment used in paints and inks. It has a rigid flat molecular structure, and in dilute solution has a low-cal xanthous color. Three polymorphs take been identified. Intermolecular hydrogen bonds are an important feature in all off these. The crystal colors range from bright cerise to violet. The anti-ulcer drug ranitidine (Zantac) was beginning patented by Glaxo-Wellcome in 1978. Seven years later a second polymorph of ranitidine was patented by the same visitor. This extended the licensing coverage until 2002, and efforts to market a generic form were thwarted, because it was not possible to prepare the offset polymorph uncontaminated by the 2d. The relatively simple aryl thiophene, designated EL1, was prepared and studied by chemists at the Eli Lilly Company. It displayed 6 polymorphic crystal forms, pictures of which are shown on the left.
| ||||||||||||||||||||||||||||||||
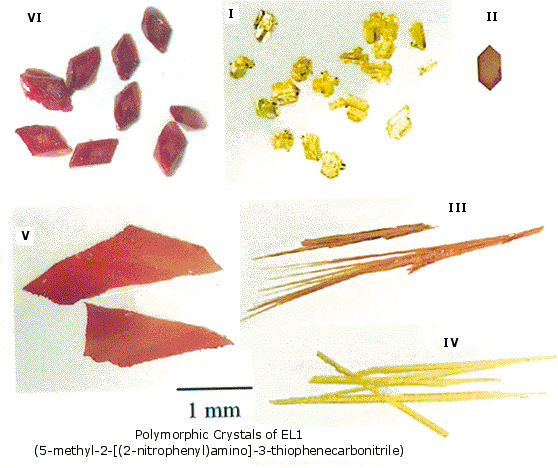 | ||||||||||||||||||||||||||||||||||
A mutual instance of changes in polymorphism is shown by chocolate that has suffered heating and/or long storage. Over time, or when information technology resets after softening, it may take white patches on it, no longer melts in your rima oris, and doesn't taste as skilful every bit information technology should. This is because chocolate has more than six polymorphs, and merely one is ideal equally a confection. It is created under advisedly-controlled factory conditions. Improper storage or transport weather condition cause chocolate to transform into other polymorphs.
Chocolate is in essence cocoa mass and carbohydrate particles suspended in a cocoa butter matrix. Cocoa butter is a mixture of triglycerides in which stearoyl, oleoyl and palmitoyl groups predominate. It is the polymorphs of this matrix that influence the quality of chocolate. Low melting polymorphs feel also viscid or thick in the mouth. Class V, the best tasting polymorph of cocoa butter, has a melting signal of 34 to 36 ºC, slightly less than the interior of the homo body, which is 1 reason information technology melts in the oral fissure. Unfortunately, the college melting grade VI is more than stable and is produced over time.
| Polymorph | Melting Indicate | Comments |
|---|---|---|
| I | 17.iv ºC | Produced by rapid cooling of a melt. |
| Ii | 23.4 ºC | Produced past cooling the melt at ii ºC/min. |
| III | 26 ºC | Produced by transformation of grade II at v-x ºC. |
| IV | 27 ºC | Produced by transformation of form III by storing at 16-21 ºC. |
| V | 34 ºC | Produced by tempering (cooling so reheating slightly while mixing). |
| VI | 36-37 ºC | Produced from V subsequently spending 4 months at room temperature. |
Water Solubility |
|---|
Solubility in Water
Water has been referred to as the "universal solvent", and its widespread distribution on this planet and essential role in life brand it the benchmark for discussions of solubility. Water dissolves many ionic salts cheers to its high dielectric constant and ability to solvate ions. The former reduces the attraction between oppositely charged ions and the latter stabilizes the ions by bounden to them and delocalizing charge density. Many organic compounds, specially alkanes and other hydrocarbons, are nigh insoluble in water. Organic compounds that are water soluble, such every bit most of those listed in the above tabular array, by and large have hydrogen bond acceptor and donor groups. The least soluble of the listed compounds is diethyl ether, which can serve only every bit a hydrogen bond acceptor and is 75% hydrocarbon in nature. Fifty-fifty so, diethyl ether is virtually two hundred times more soluble in water than is pentane.
The chief characteristic of water that influences these solubilities is the extensive hydrogen bonded association of its molecules with each other. This hydrogen bonded network is stabilized past the sum of all the hydrogen bail energies, and if nonpolar molecules such as hexane were inserted into the network they would destroy local structure without contributing whatsoever hydrogen bonds of their own. Of course, hexane molecules experience significant van der Waals attraction to neighboring molecules, but these bonny forces are much weaker than the hydrogen bail. Consequently, when hexane or other nonpolar compounds are mixed with water, the stiff association forces of the water network exclude the nonpolar molecules, which must then exist in a dissever phase. This is shown in the following illustration, and since hexane is less dense than water, the hexane phase floats on the water phase.
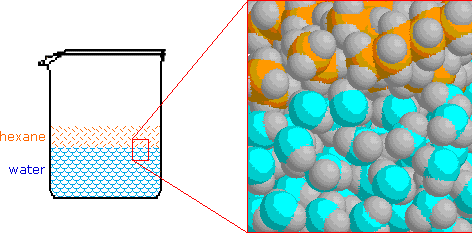
Information technology is important to think this trend of water to exclude nonpolar molecules and groups, since it is a factor in the structure and behavior of many complex molecular systems. A common nomenclature used to describe molecules and regions within molecules is hydrophilic for polar, hydrogen bonding moieties and hydrophobic for nonpolar species.
| |
|---|
Do Problems |
|---|
This page is the property of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to aid in capacity building in chemical education. 05/05/2013
| Return to Table of Contents |
|---|
![]()
Terminate of this supplementary topic
![]()
More about Intermolecular Forces |
|---|
Intermolecular Forces and Physical Properties
The attractive forces that exist between molecules are responsible for many of the bulk concrete properties exhibited by substances. Some compounds are gases, some are liquids, and others are solids. The melting and humid points of pure substances reverberate these intermolecular forces, and are commonly used for identification. Of these two, the humid point is considered the nigh representative measure of full general intermolecular attractions. Thus, a melting point reflects the thermal energy needed to convert the highly ordered array of molecules in a crystal lattice to the randomness of a liquid. The distance between molecules in a crystal lattice is small and regular, with intermolecular forces serving to constrain the motion of the molecules more severely than in the liquid country. Molecular size is of import, but shape is also critical, since individual molecules need to fit together cooperatively for the bonny lattice forces to exist large. Spherically shaped molecules generally accept relatively high melting points, which in some cases approach the boiling point, reflecting the fact that spheres can pack together more closely than other shapes. This construction or shape sensitivity is 1 of the reasons that melting points are widely used to identify specific compounds.
Boiling points, on the other hand, substantially reverberate the kinetic energy needed to release a molecule from the cooperative attractions of the liquid state so that it becomes an unencumbered and relative independent gaseous state species. All atoms and molecules have a weak attraction for i another, known as van der Waals attraction. This attractive strength has its origin in the electrostatic attraction of the electrons of one molecule or atom for the nuclei of another, and has been called London dispersion strength.
The following animation illustrates how close arroyo of two neon atoms may perturb their electron distributions in a manner that induces dipole attraction. The induced dipoles are transient, but are sufficient to permit liquefaction of neon at low temperature and high pressure.

In full general, larger molecules have higher boiling points than smaller molecules of the same kind, indicating that dispersion forces increase with mass, number of electrons, number of atoms or some combination thereof. The following table lists the humid points of an assortment of elements and covalent compounds equanimous of molecules lacking a permanent dipole. The number of electrons in each species is noted in the showtime column, and the mass of each is given as a superscript number preceding the formula.
| # Electrons | Molecules & Boiling Points ºC |
|---|---|
| 10 | 20Ne –246 ; 16CHfour –162 |
| 18 | 40Ar –186 ; 32SiH4 –112 ; thirtyC2Hsix –89 ; 38F2 –187 |
| 34-44 | 84Kr –152 ; 58C4Hten –0.5 ; 72(CH3)4C 10 ; 71Cltwo –35 ; 88CFfour –130 |
| 66-76 | 114[(CH3)3C]two 106 ; 126(CHtwo)ix 174 ; 160Br2 59 ; 154CCl4 77 ; 138C2F6 –78 |
Two ten electron molecules are shown in the first row. Neon is heavier than methyl hydride, merely it boils 84º lower. Methane is equanimous of 5 atoms, and the additional nuclei may provide greater opportunity for induced dipole germination equally other molecules approach. The ease with which the electrons of a molecule, atom or ion are displaced by a neighboring charge is called polarizability, so we may conclude that marsh gas is more polarizable than neon. In the second row, four 18 electron molecules are listed. Nearly of their boiling points are higher than the 10 electron compounds neon and methane, but fluorine is an exception, boiling 25º below methane. The remaining examples in the table suit to the correlation of humid point with total electrons and number of nuclei, but fluorine containing molecules remain an exception.
The anomalous beliefs of fluorine may exist attributed to its very high electronegativity. The fluorine nucleus exerts such a strong attraction for its electrons that they are much less polarizable than the electrons of most other atoms.
Of course, boiling indicate relationships may be dominated by even stronger attractive forces, such as those involving electrostatic attraction betwixt oppositely charged ionic species, and betwixt the partial charge separations of molecular dipoles. Molecules having a permanent dipole moment should therefore have college boiling points than equivalent nonpolar compounds, as illustrated by the data in the post-obit table.
| # Electrons | Molecules & Humid Points ºC |
|---|---|
| 14-18 | thirtyC2Hvi –89 ; 28H2C=CH2 –104 ; 26HC≡CH –84 ; 30H2C=O –21 ; 27HC≡North 26 ; 34CH3-F –78 |
| 22-26 | 42CH3CH=CH2 –48 ; 40CH3C≡CH –23 ; 44CH3CH=O 21 ; 41CH3C≡N 81 ; 46(CH3)iiO –24 ; l.fiveCH3-Cl –24 ; 52CH2Ftwo –52 |
| 32-44 | 58(CH3)iiiCH –12 ; 56(CHthree)2C=CHii –7 ; 58(CH3)2C=O 56 ; 59(CHthree)3Northward 3 ; 95CH3-Br 45 ; 85CH2Cl2 40 ; lxxCHF3 –84 |
In the first row of compounds, ethane, ethene and ethyne take no molecular dipole, and serve every bit useful references for unmarried, double and triple bonded derivatives that do. Formaldehyde and hydrogen cyanide clearly bear witness the enhanced intermolecular attraction resulting from a permanent dipole. Methyl fluoride is anomalous, equally are nigh organofluorine compounds. In the second and 3rd rows, all the compounds have permanent dipoles, but those associated with the hydrocarbons (first two compounds in each case) are very pocket-sized. Big molecular dipoles come chiefly from bonds to loftier-electronegative atoms (relative to carbon and hydrogen), especially if they are double or triple bonds. Thus, aldehydes, ketones and nitriles tend to be higher boiling than equivalently sized hydrocarbons and alkyl halides. The atypical behavior of fluorine compounds is unexpected in view of the large electronegativity difference between carbon and fluorine.
Hydrogen Bonding
About of the simple hydrides of group IV, Five, VI & 7 elements brandish the expected rise in boiling indicate with number of electrons and molecular mass, merely the hydrides of the nearly electronegative elements (nitrogen, oxygen and fluorine) have abnormally loftier boiling points, depicted earlier as a graph, and besides listed on the right.
| Grouping | Molecules & Humid Points ºC |
|---|---|
| Seven | HF 19 ; HCl –85 ; HBr –67 ; Hello –36 |
| Vi | HtwoO 100 ; HtwoS –60 ; H2Se –41 ; HiiTe –2 |
| V | NHthree –33 ; PH3 –88 ; AsH3 –62 ; SbHiii –18 |
Organic compounds incorporating O-H and Northward-H bonds will also exhibit enhanced intermolecular attraction due to hydrogen bonding. Some examples are given below.
| Grade | Molecules & Boiling Points ºC | |
|---|---|---|
| Oxygen Compounds | C2H5OH 78 ; (CHiii)twoO –24 ; (CH2)2O xi ethanol dimethyl ether ethylene oxide | (CH2)3CHOH 124 & (CH2)4O 66 cyclobutanol tetrahydrofuran |
| Nitrogen Compounds | C3H7NH2 l ; CiiHfiveNH(CH3) 37 ; (CHiii)threeN three propyl amine ethyl methyl amine trimethyl amine | (CHtwo)4CHNH2 107 & (CHtwo)4NCHthree fourscore cyclopentyl amine Northward-methylpyrrolidine |
| Complex Functions | CiiHvCO2H 141 & CH3COtwoCH3 57 propanoic acid methyl acetate | C3H7CONH2 218 & CH3CON(CHthree)2 165 butyramide N,N-dimethylacetamide |
![]()
H2o Solubility
Water is the single most arable and important liquid on this planet. The miscibility of other liquids in water, and the solubility of solids in water, must be considered when isolating and purifying compounds. To this end, the following table lists the h2o miscibility (or solubility) of an array of low molecular weight organic compounds. The influence of the important hydrogen bonding atoms, oxygen and nitrogen is immediately apparent. The beginning row lists a few hydrocarbon and chlorinated solvents. Without exception these are all immiscible with h2o, although it is interesting to note that the π-electrons of benzene and the nonbonding valence electrons of chlorine human action to slightly increase their solubility relative to the saturated hydrocarbons. When compared with hydrocarbons, the oxygen and nitrogen compounds listed in the second, third and fourth rows are over a hundred times more soluble in h2o, and many are completely miscible with water.
| Compound Blazon | Specific Compounds | Grams/100mL | Moles/Liter | Specific Compounds | Grams/100mL | Moles/Liter | |
|---|---|---|---|---|---|---|---|
| Hydrocarbons & Alkyl Halides | butane hexane cyclohexane | 0.007 0.0009 0.006 | 0.0012 0.0001 0.0007 | benzene methylene chloride chloroform | 0.07 i.50 0.8 | 0.009 0.180 0.07 | |
| Compounds Having Ane Oxygen | 1-butanol tert-butanol cyclohexanol phenol | 9.0 complete 3.6 8.7 | 1.ii consummate 0.36 0.ninety | ethyl ether THF furan anisole | 6.0 consummate ane.0 1.0 | 0.80 complete 0.xv 0.09 | |
| Compounds Having Ii Oxygens | 1,3-propanediol 2-butoxyethanol butanoic acid benzoic acid | complete consummate complete consummate | complete complete complete complete | 1,ii-dimethoxyethane 1,4-dioxane ethyl acetate γ-butyrolactone | complete complete viii.0 complete | consummate complete 0.91 consummate | |
| Nitrogen Containing Compounds | 1-aminobutane cyclohexylamine aniline pyrrolidine pyrrole | complete complete iii.4 complete 6.0 | complete consummate 0.37 complete 0.9 | triethylamine pyridine propionitrile ane-nitropropane DMF | 5.5 complete 10.3 i.5 complete | 0.54 complete 2.0 0.17 complete |
Some general trends are worth noting from the data higher up. Kickoff, alcohols (second row left cavalcade) are unremarkably more soluble than equivalently sized ethers (second row right column). This reflects the fact that the hydroxyl group may role as both a hydrogen bond donor and acceptor; whereas, an ether oxygen may serve only as an acceptor. The increased solubility of phenol relative to cyclohexanol may exist due to its greater acidity also as the pi-electron effect noted in the first row.
The cyclic ether THF (tetrahydrofuran) is more than soluble than its open chain analog, peradventure considering the oxygen atom is more attainable for hydrogen bonding to water molecules. Due to the decreased basicity of the oxygen in the aromatic chemical compound furan, it is much less soluble. The oxygen atom in anisole is likewise deactivated past conjugation with the benzene band (note, information technology activates the ring in electrophilic substitution reactions). A 2nd oxygen cantlet dramatically increases water solubility, as demonstrated past the compounds listed in the third row. Once more hydroxyl compounds are listed on the left.
Nitrogen exerts a solubilizing influence similar to oxygen, as shown by the compounds in the fourth row. The primary and secondary amines listed in the left manus column may function as both hydrogen bond donors and acceptors. Aromaticity decreases the basicity of pyrrole, but increases its acidity. The compounds in the right column are simply capable of an acceptor role. The low solubility of the nitro compound is surprising.
This page is the belongings of William Reusch. Comments, questions and errors should be sent to whreusch@msu.edu.
These pages are provided to the IOCD to assist in capacity edifice in chemic pedagogy. 05/05/2013
![]()
Finish of this supplementary topic
![]()
Source: https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/physprop.htm
Enregistrer un commentaire for "what happens to bonds between molecules when a substance melts"